
PLASMOQUINE
A trusted Schedule 4 medicine in South Africa
Schedule 4 medicine in South Africa, available by prescription only

About Plasmoquine
Product Information
PLASMOQUINE (chloroquine sulphate) is a Schedule 4 medicine in South Africa. It is available only with a prescription from an authorized healthcare provider.
Registration Number: Z/20.2.6/127
Classification: S4 Treatment
Registered with the South African Health Products Regulatory Authority
Important Information
- 1
Prescription Only: Plasmoquine is a Schedule 4 medicine that requires a prescription from an authorized healthcare provider.
- 2
Medical Supervision: This medication should only be taken under the supervision of a healthcare professional.
- 3
Documentation: Please review the Patient Information Leaflet for complete information about this medication.
Download Resources
Product Label Information
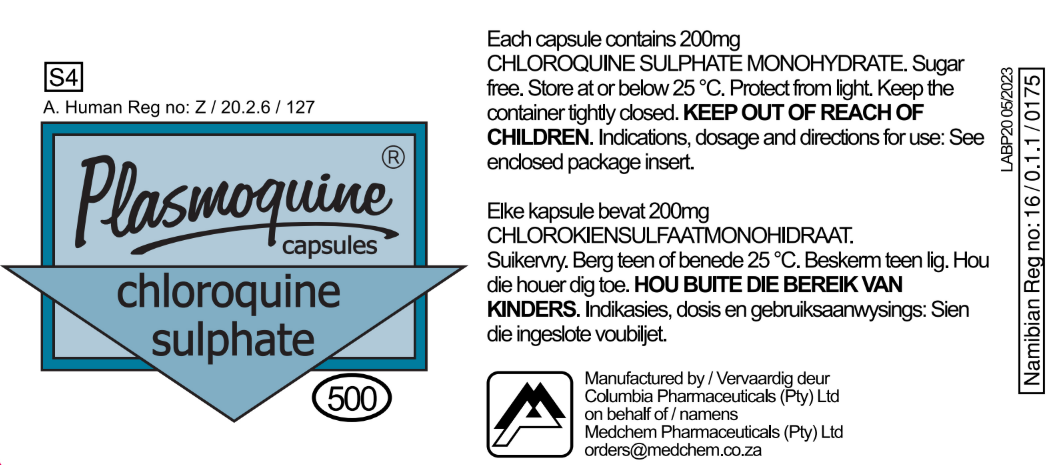
Label Details
- Active Ingredient: 200mg CHLOROQUINE SULPHATE MONOHYDRATE per capsule
- Registration: Z/20.2.6/127
- Schedule: S4 (Prescription only)
- Storage: Below 25°C, protected from light
Adverse Events Reporting
Patient safety is our priority. If you experience any adverse events while taking PLASMOQUINE, please follow these steps.
Immediate Action
If you experience any severe adverse events, contact your doctor immediately or visit the nearest medical center.
Medical Consultation
Consult with the healthcare professional who prescribed Plasmoquine. They can provide guidance on managing side effects.
Report to Medchem
Once you are safe, please report any adverse events to us. This helps us improve our products and report to SAHPRA.
You can also report side effects to SAHPRA via the Med Safety APP (Medsafety X SAHPRA) and eReporting platform (whoumc.org) found on the SAHPRA website.
By reporting side effects, you can help provide more information on the safety of PLASMOQUINE.
Report Adverse EventRegulatory Compliance
Medchem Pharmaceuticals adheres to strict regulatory standards to ensure the safety and efficacy of our products.

Registration & Licensing
Plasmoquine is registered with SAHPRA with complete safety, quality, and efficacy data
Medchem holds a valid Establishment License issued by SAHPRA's Inspectorate branch
Our manufacturing facilities comply with Good Manufacturing Practices (GMP)
Pharmacovigilance & Quality
Comprehensive pharmacovigilance system to monitor and report adverse events
Regular quality assurance inspections and compliance with SAHPRA requirements
Plasmoquine is a Schedule 4 medicine, available only with a prescription from an authorized healthcare provider
Have Questions About PLASMOQUINE?
Our team is ready to assist you with any inquiries about our products. Contact us today for more information.
Contact Us